Understanding Test Results
On November 1st, 2020, the Arizona Department of Health Services (AZDHS) began requiring testing of Marijuana flower, concentrates, and infused products (e.g., edibles, beverages, topicals). The testing program adopted and refined a set of industry-standard testing practices, testing laboratory certification, testing protocols, and safety standards for both Adult Use and Medical programs. Marijuana product test results can be overwhelming in their detail and complexity. This brief guide is meant to assist our wholesale dispensary customers and their customers & patients in making sense of these reports.

AZDHS Marijuana Testing Requirements
Per Arizona Senate Bill 1494 (SB-1494), the following five (5) types of analysis must be performed on all cannabis and cannabis infused products. These tests include:
Pesticides - Chemical compounds sometimes used in the cultivation of Marijuana Flower. Generally expunged from the plant during the final stages of cultivation.
Residual Solvents - Compounds, frequently a type of hydrocarbon such as highly-purified butane or propane, or CO2, or other solvents used to strip the trichomes (and their cannabinoids) from the Flower material being processed. Residual solvent is generally removed through a heating/vacuum process.
Heavy Metals - Elements found in soil and other growth mediums that can inadvertently be taken up by the plant where it remains.
Microbials - Mold and bacteria or toxic compounds they create can be present on cannabis products. The significant presence of these compounds may imply poor sanitation and/or handling practices.
Potency - Defined as the amount of Total THC (THCa+THC), and Total CBD (CBDa+CBD) present in the product.
Safety limits for each of these areas are established and only products that PASS all tests are considered legal for sale in Arizona. Examples of annotated testing panels for each area are provided further in this document. For additional information including updated testing rules, certified laboratories, etc., please refer to azdhs.com/marijuana.
How Halo Infusions Tests Products
Halo Infusions tests our Topical and Edible Products by first testing the Marijuana Source Material for Pesticides, Residual Solvents, and Heavy Metals. Performing these tests on the Flower or Extract-based Marijuana Source Materials used for infusions eliminates the need to perform these tests on the Final Product. Once a product has completed manufacturing (Final Product), testing for Microbials, and THC & CBD Potency are performed.*
Halo Infusions tests our Topical and Edible Products by first testing the Marijuana Source Material for Pesticides, Residual Solvents, and Heavy Metals. Performing these tests on the Flower or Extract-based Marijuana Source Materials used for infusions eliminates the need to perform these tests on the Final Product. Once a product has completed manufacturing (Final Product), testing for Microbials, and THC & CBD Potency are performed.*
Halo Infusions tests our Topical and Edible Products by first testing the Marijuana Source Material for Pesticides, Residual Solvents, and Heavy Metals. Performing these tests on the Flower or Extract-based Marijuana Source Materials used for infusions eliminates the need to perform these tests on the Final Product. Once a product has completed manufacturing (Final Product), testing for Microbials, and THC & CBD Potency are performed.*
Testing Summary Page
To simplify intake processing of Halo Infusions’ products by our wholesale Dispensary customers, Halo Infusions provides a Summary Cover Sheet with all product batches at delivery. The delivery form includes all testing categories and their test status.
This presence of this form represents that Halo Infusions warranties this product batch to fully conform to all AZ Marijuana Testing Requirements and is therefore suitable for sale.
Wholesale Dispensary staff and Patients/Adult Use Customers may also enter the product’s Batch Number directly into our testing database at haloinfusions.com/testing, to obtain complete testing reports at Point of Sale (POS) stations, or from any Internet-connected device.
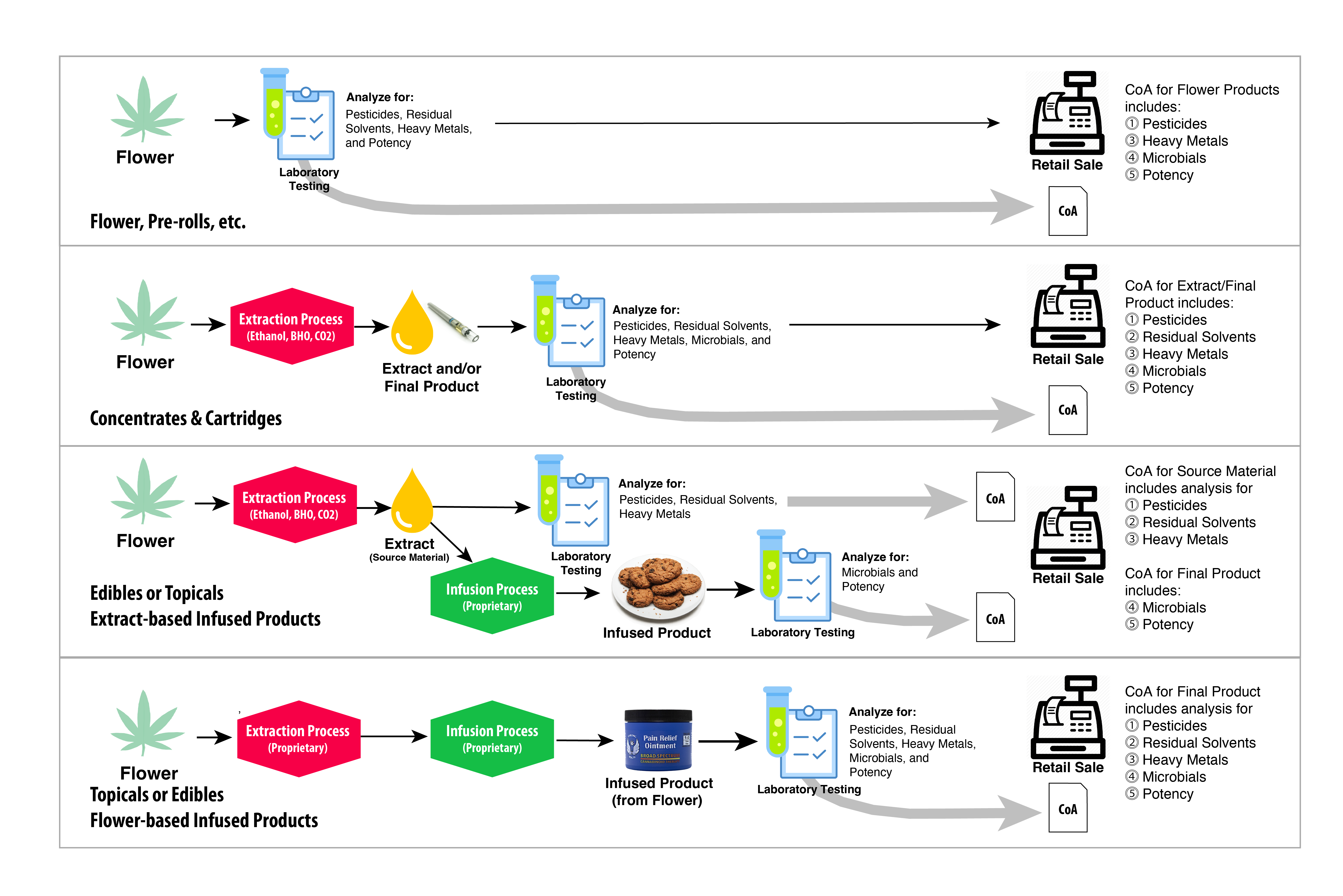
* This results in two (2) or more COAs being supplied with each product batch that Halo Infusions produces. This approach is fully compliant with R9-17-317.01 Analysis of Medical Marijuana or a Marijuana Product and R9-18-311 Analysis of Marijuana or a Marijuana Product . The figure below illustrates the testing workflow approved by AZDHS.
The illustration shows the testing process for Marijuana and Marijuana-infused products. The top-most row shows Flower products, while the second row shows Concentrates & Cartridges. The final two rows (Row 3 and 4) show the testing workflow for infused products (using either Extract or Flower).

How to Read Test Results
Test results are typically two or more pages in length and include panels which provide both summary and detailed information. Common terminology includes:
Crude - Raw, or unrefined Crude Cannabis (Marijuana) Oil.
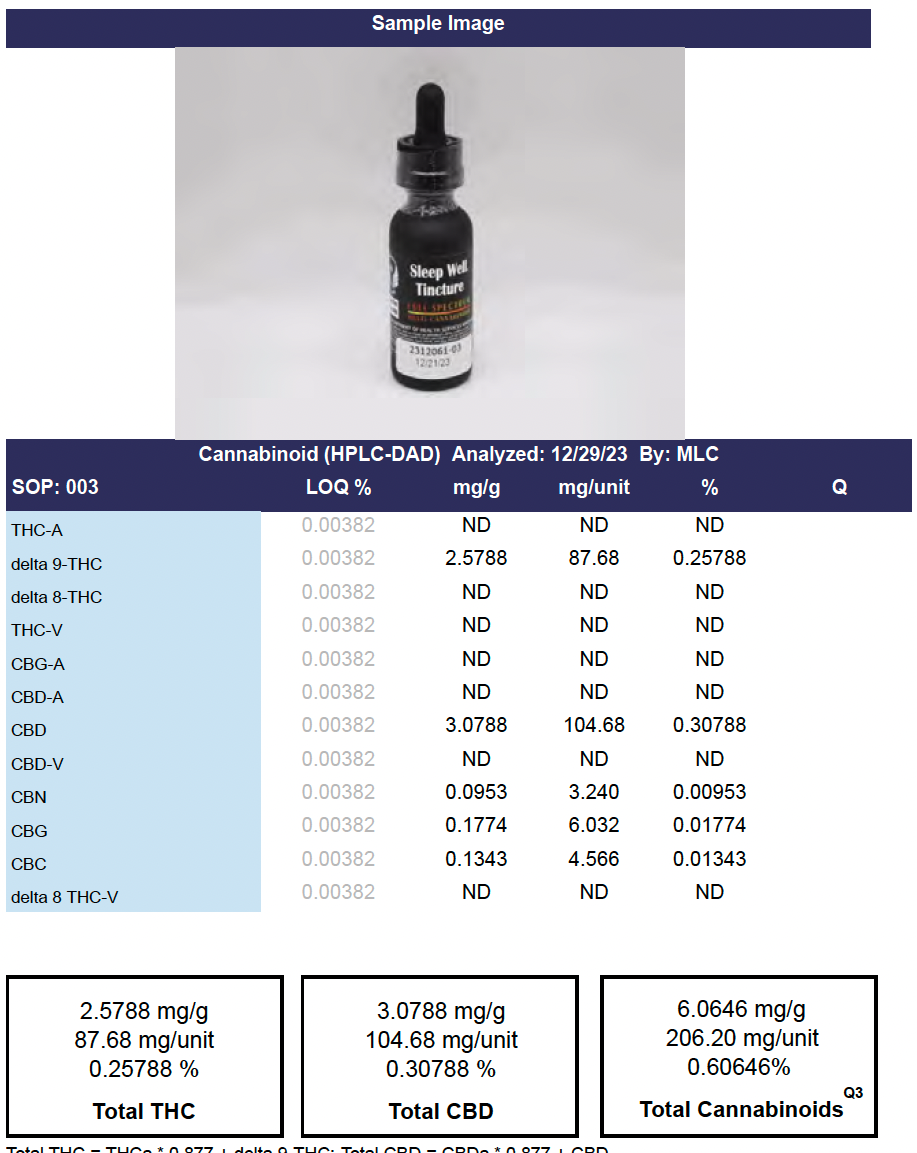
Total THC - Calculated total amount of THC present (THCa x .877 + ∆9 THC)+
Total CBD - Calculated total amount of CBD present (CBDa x .877 + ∆9 THC)+
Total Cannabinoids - Total percentage presence of all cannabinoids tested.
LOQ - Limit of Quantitation. Defines the limit to which the equipment can detect a compound’s presence.
<LOQ - Indicates the compound was detected but the amount is too small to characterize.
ND - None Detected. The compound was not found in testing.
NR - Not Reported. The compound was not found in testing.
Analyte - A substance whose chemical constituents are being identified and measured.
Batch # - The principal identifier for the product tested. The Batch # allows for seed-to-sale tracking of the Marijuana product tested.
PPM - Parts per Million. Defines the concentration of the compound tested per a unit volume. PPM is typically used as a measure of the presence of an undesirable substance and the limits at which it becomes toxic.
mg/g - Milligrams per gram. Typically references the amount of a compound (in milligrams) detected per gram of sample.
mg/unit - Milligrams per unit. Same as above, but calculated as total in product package.
Resin - A concentrated form of the active ingredients in Marijuana, especially cannabinoids & terpenes. Unusually produced through an extraction process that includes one or more of pressure, heat, and/or solvents.
Pathogen - A (potentially) infectious organism that causes disease.
Source Material Test Panels
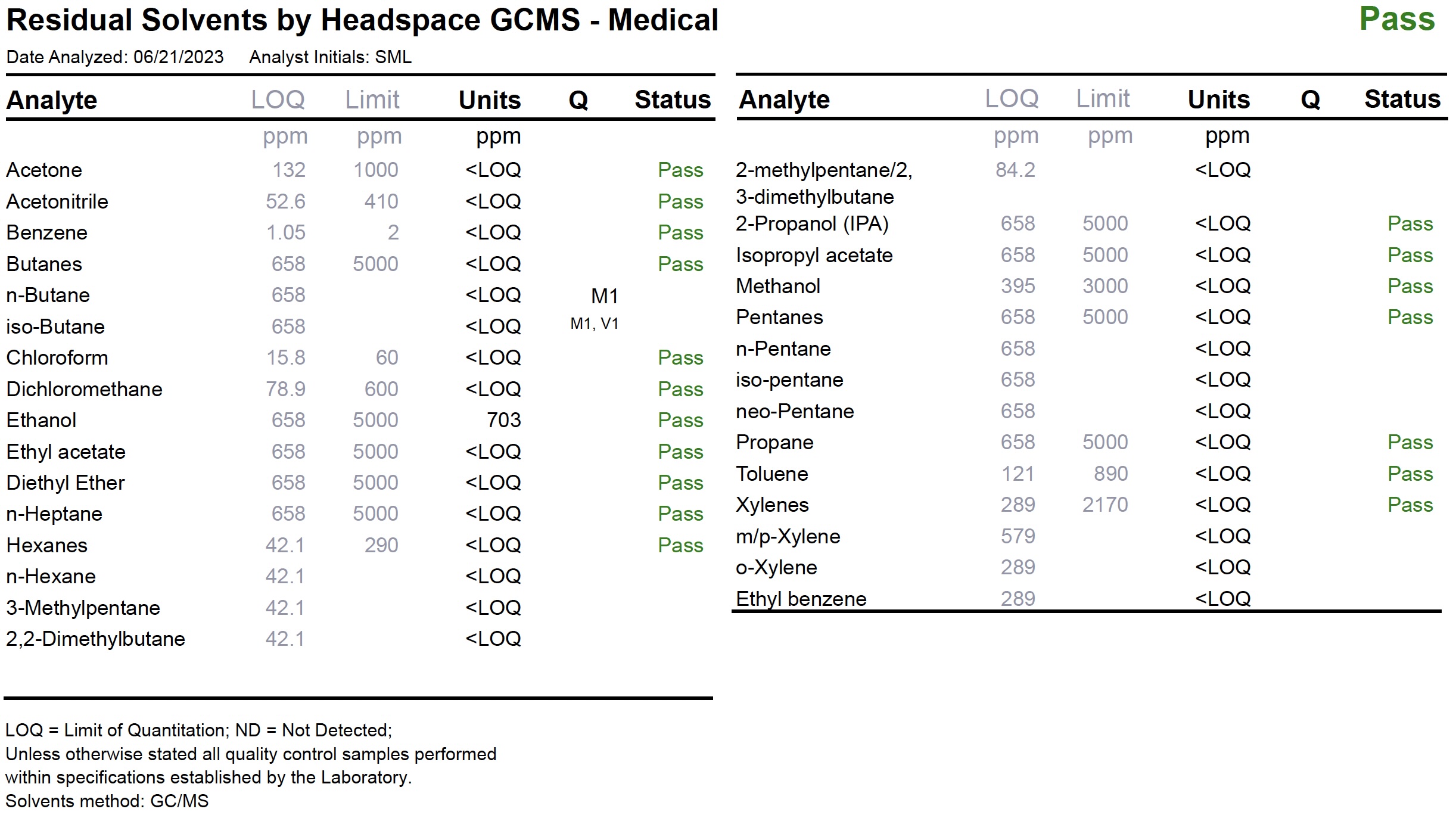
Residual Solvents
Most concentrates, whether used directly for inhalation, or as an ingredient in edibles and topicals, are processed using a solvent such as CO2, butane, propane, other hydrocarbons, water, or with our products, ethanol to extract the cannabinoids and terpenes from the plant material. The figure below illustrates a Test Panel for Residual Solvents.
In some cases, the solvent and impurities from the solvent remain in the extracted material. These are called residual solvents and are the byproducts of the extraction process. Safety standards require that the presence of residual solvents fall below designated limits to be eligible for sale. Some concentrate products can be remediated by additional processing.
Per AZDHS testing rules, when reporting test results for Source Material, one (1) COA may be submitted together with a second (2) COA for Microbials and Potency.
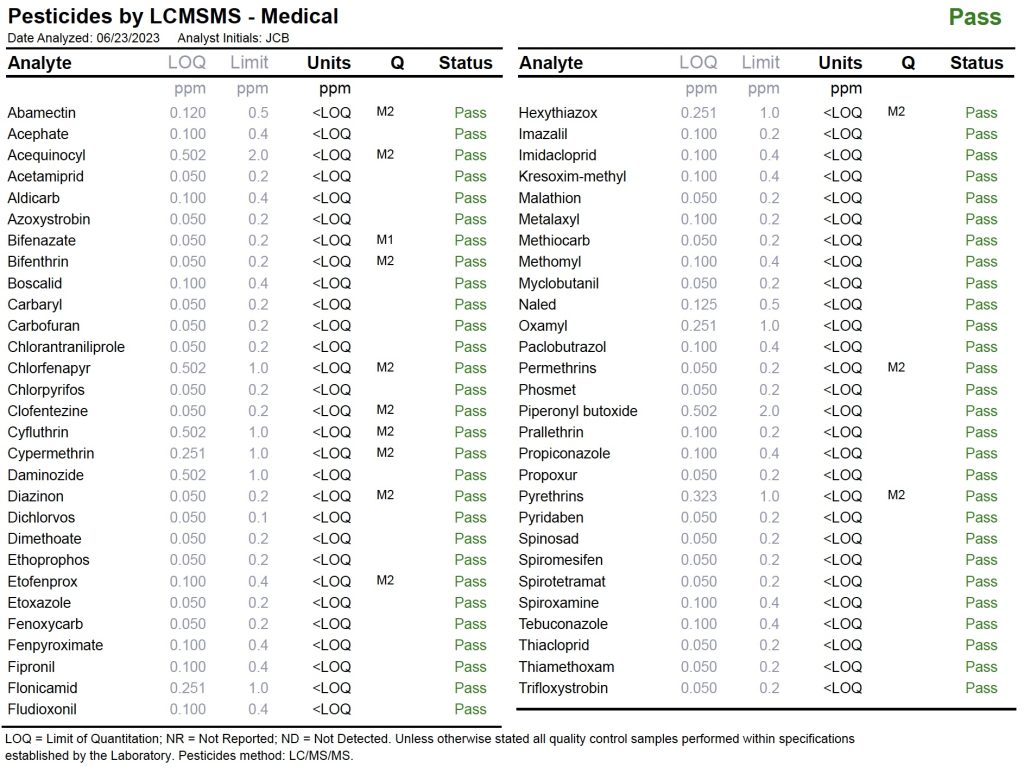
Pesticides
Pesticides are man-made complex compounds, used to repel or eradicate insects, rodents and other pests. While many pesticides become inert after application, some persist on plants and in their soil.
Dozens of common pesticides that may be used by cultivators are tested and any quantities above threshold are reported. Only safe products are permitted for sale. The figure below provides an example of a Test Panel for Pesticides.
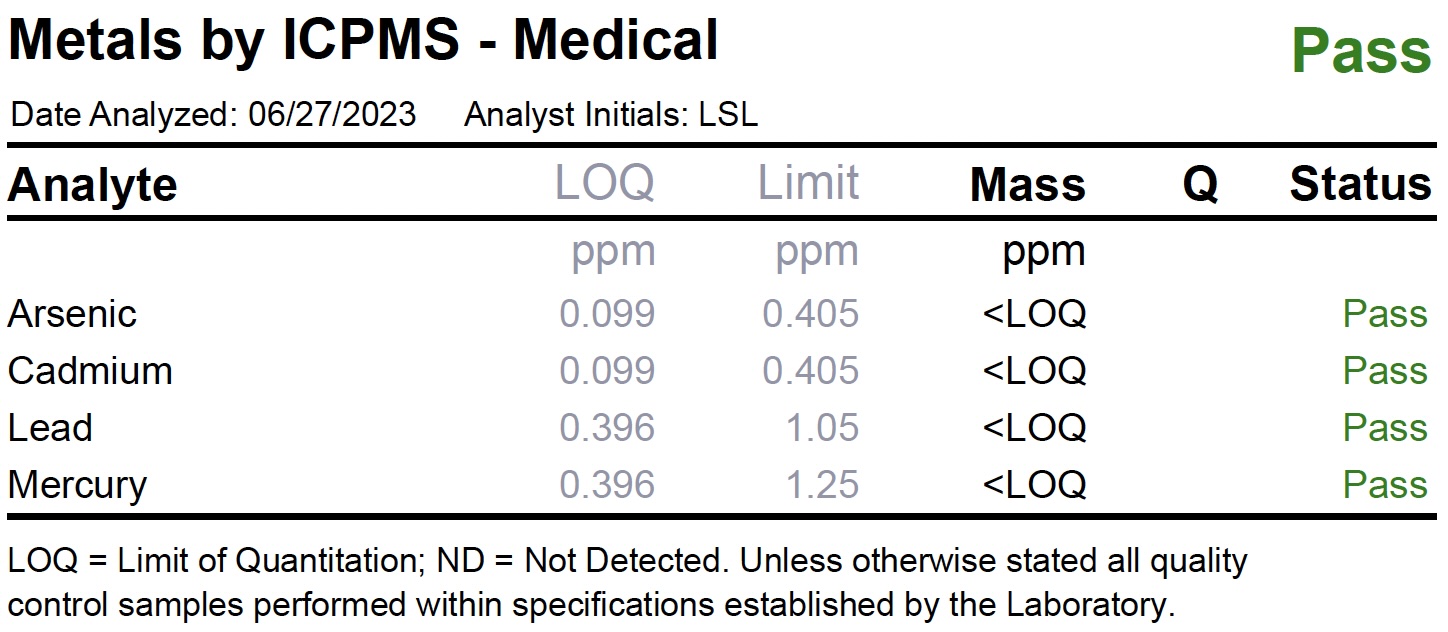
Heavy Metals
Heavy metals are metallic chemical elements that have a relatively high density and can be toxic or poisonous even at low concentrations. Heavy metals occur in cannabis products when the plant uptakes these elements while in cultivation, or when heavy metal-containing nutrients are used. The figure below illustrates a Heavy Metals Test Panel.
Final Product Test Panels
Final Product Testing: Potency & Microbials
Final products are always tested for Microbials at the Final Product packaging stage to ensure safety. Cannabinoid Potency analyses are performed to meet AZDHS requirements for THC and CBD product label claims and to ensure Medical and Adult Use consumption rules are followed. The figure that follows is Final Product Summary Test Panel, which includes cannabinoid expression.
Potency
The Potency Test Panel is typically the first panel displayed in test results. It includes information on Cannabinoids detected, most notably THC and CBD.
Other Cannabinoids and their acidic pre-cursors (THCa, CBDa, CBGa, etc.) are also usually tested and reported.
Cannabinoids are the principal active compounds found in Marijuana, and are largely regarded as responsible for the majority of its effects, including euphoria and pain relief, or having antibiotic, anticonvulsant, or anti-inflammatory properties.
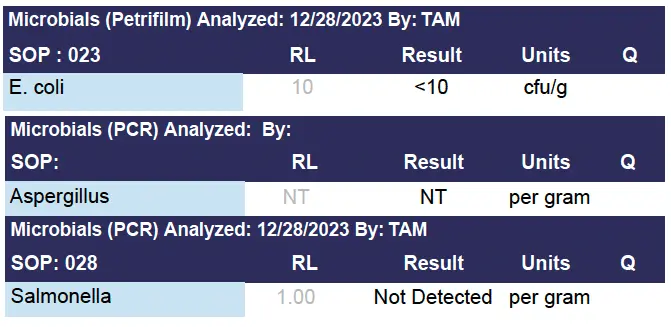
Microbials
E. Coli and Salmonella are bacteria found in the environment, foods, and intestines of people and animals. Most types of E. Coli are harmless or cause relatively brief symptoms. A few strains of E. Coli and Salmonella can be pathogenic. A mycotoxin is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. The figure below shows a Test Panel for Microbials.
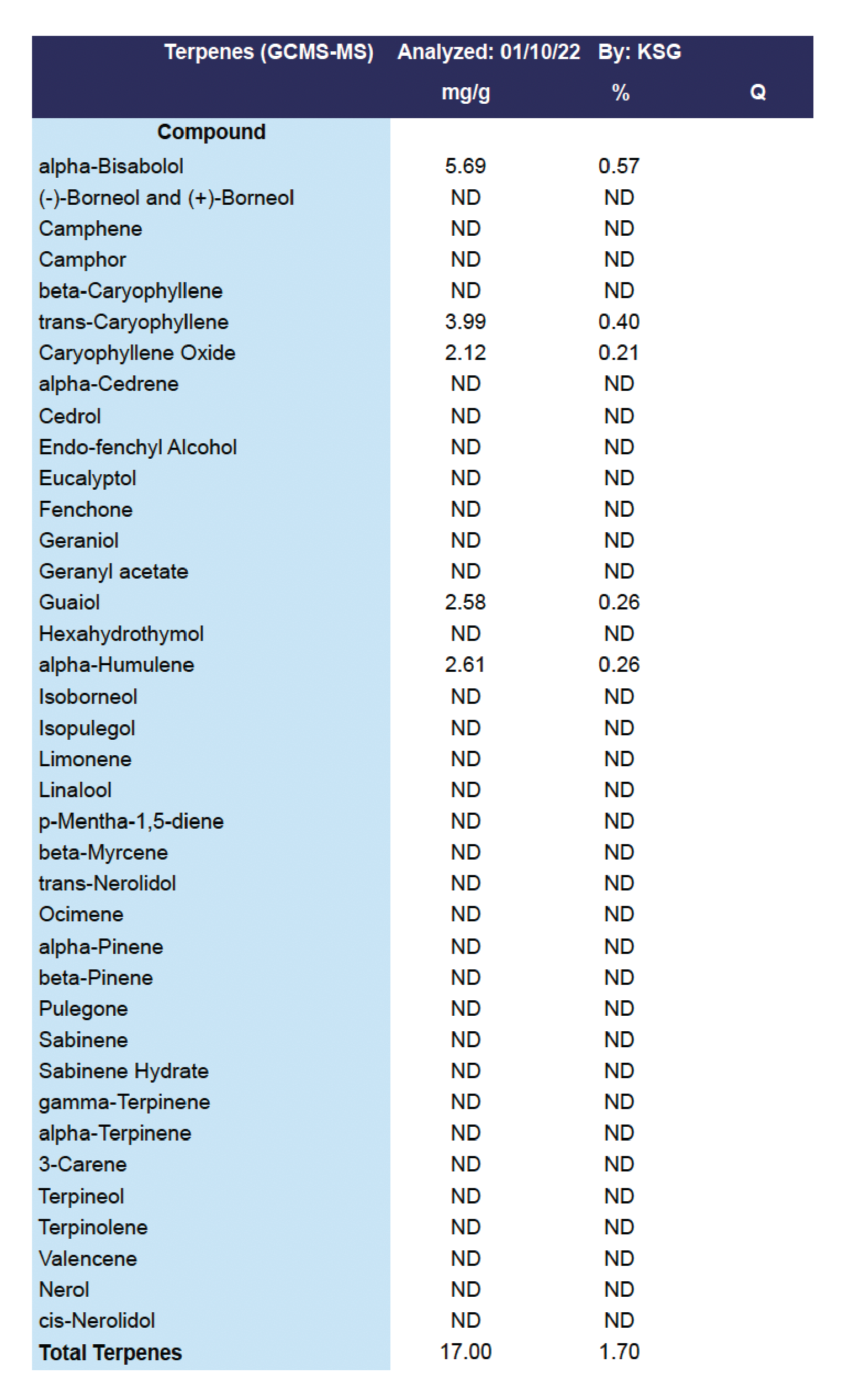
Terpene Test Panel
The second panel usually found in Flower, Concentrate and Cartridge reports is a Terpene Test Panel. This panel includes any test data reported for terpenes.
Terpenes are important aromatic compounds originating from the same trichome sources as cannabinoids. Terpenes directly impact the taste and smell of smoked Marijuana and are believed to be a key component of the Entourage Effect.
Testing for Terpenes is NOT a requirement of the AZDHS. Terpene testing is usually done when a cultivator or manufacturer wants to make products that incorporate terpenes.
The figure below provides a Test Panel for Terpenes.
Halo Infusions Testing
All of our test results can be found conveniently at our website haloinfusions.com/testing. All that is required is to enter the Batch ID into the provided field and Voila! You will find the results related to your product with easy to navigate buttons that detail which results you are reviewing. There is also a zip file download for the ease of capturing all related results with one click of a button.
When reading our Certificates of Analysis (COAs), notice there are typically 2-3 CoAs in our testing packet. In this you will find the Final Product analysis (Potency and Microbials), the Source Material analysis (Pesticides, Residual Solvents and Heavy Metals) and when applicable, the Intermediary dosing material analysis (Potency and Microbials, to ensure dosing accuracy and sanitation control through processing).
Additional Product COAs
When products do not pass muster for Potency (+/- 20% of the lab reported value) with results from the lab, the state has allowed for testing at two other labs. This allows for the potential uncertainty from the first lab to not hinder the ability to sell products that may otherwise be dosed correctly.
In these situations you will find that we list two additional COAs in our testing packet, Primary Retest and Secondary Retest. We label the product with one of the two passing retest potency results; this is typically the potency result closest to the target on our label.
For More Information
For more information on product testing and reporting, please contact our Customer Care team at customercare@haloinfusions.com.
